101,156 SUSPECTED AEFI (2,380 DEATHS) FOLLOWING COVID-19 VACCINATION IN THE PHILIPPINES AS OF 29 MAY 2022
Class 1 Drug Recall is applicable when there is reasonable probability that a product is associated with serious adverse health consequence or death. Why isn't this applied to the EUA C-19 Vaccines?
The Philippines Food and Drug Administration (FDA) replaced the original BFAD (Bureau of Food and Drugs) on August 18, 2009. This new agency, under Republic Act 9711 was empowered to seize health products found violating the law without any court order. FDA director Nazarita Tacandong said RA 9711 would “help eliminate unregistered and unsafe products in the market”… “help strengthen our pharmaco-vigilance,” and “we’ll be able to make sure that only the safe and quality medicine will be out in the market.”
The original BFAD issued Bureau Circular No. 8, series of 2001, setting out the guidelines for product recall “to insure safe and good quality supply of food, drugs and cosmetics and to regulate the production, sale and traffic of the same to protect the health of the people”.
A Class 1 Recall is used in a situation where there is a reasonable probability that the use of a violative product will cause serious adverse health consequences or death.
Note the wording under 5.1 “reasonable probability”, and “serious adverse health consequences or death” being basis for Class 1 Recall.
This was strengthened under Bureau Circular 2012-012 which provided guidelines for handling rapid alerts arising from quality defects. It also covers investigational product during clinical trials, which is directly applicable to the Covid-19 Vaccines.
With the issue of Emergency Use Authorization (EUA) for the Covid-19 Vaccines, guidelines here, the Philippines FDA was tasked with Pharmacovigilance and safety monitoring. They produce a weekly report on Suspected Adverse Events Following Covid-19 Vaccination. This report can be hard to find online even if one enters exactly the right name. This should be the link to the latest FDA report as of 29th May 2022, but the link doesn’t work consistently. Luckily I downloaded it already.
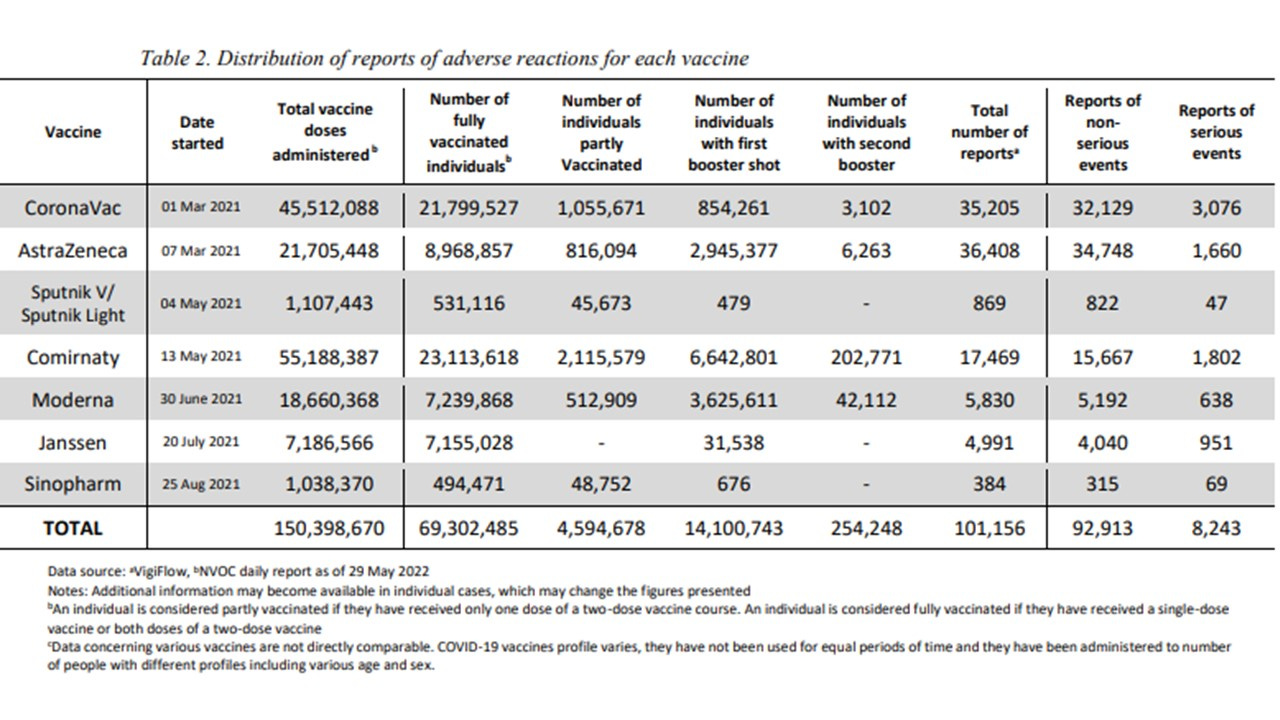
This latest report identifies that 150,398 million doses of vaccine have been delivered (an increase of 1,026,928 doses since 22nd of May) making 69,302,485 Filipinos fully dosed, 4,595,678 partly dosed, and 14,354,991 having received 2nd and/ or 1st boosters.
This latest report presents 101,156 adverse reactions of which 8,243 are considered serious (increase of 168 since 22nd May), and including 2,380 deaths (increase of 51 since 22nd May). These deaths and injuries are surely massively underreported (from international literature) by a magnitude of at least 41, possibly 42.3 and likely even to more than 100x for milder reactions. Bear these reports in mind as you read the intent and the conclusions drawn below!
The About section on Page 2 of the report states a noble aim of identifying “signal” if any exists:-
The Summary section on page 3, yet again states there is no basis for revising the current use of covid-19 vaccines, and reassures that a reported adverse reaction does not mean that a vaccine caused that reaction:-
DOH-FDA, how can these products be considered safe and effective for use? How can the public be reassured considering that you have 101,156 reported adverse reactions with 8,243 serious reactions, and 2,380 reports of death (including 37 children, and at least 2 pregnant women (from FOI request))?
Would you consider that these pharmacovigilance reports are evidence of reasonable probability that these products are associated with serious adverse health consequence or death?
At what point will a signal be called? Can you give a number? 2,380 deaths are not enough? Given the known under reporting the deaths could well be 97,580 (41x) or more, including 1,517 children. This figure is 2.4x lower than the Philippines preliminary 2021 excess all cause mortality of 232,360 for which there is no possible rational explanation except the vaccines, and maybe the vaccine accelerated covid-19 cases and deaths. 101,156 AEFI could well be more than 10 million.
The Philippines have sufficient regulations in place to enable / cover recall of products found to be unsafe! With these numbers of reports of adverse reactions following vaccination, surely it has already been established that there is reasonable probability of serious adverse consequence or death from the Covid-19 vaccines.
Covid-19 Vaccines are both unregistered (EAU is not ‘registration’) and unsafe! Will any party be prepared to instigate a CLASS 1 Recall following government guidelines?








This argument/information must be submitted FOR THE RECORD to Congress as the constitutional oversight body and the members thereof be reminded of their responsibility to their constituents, WE THE PEOPLE, who have been adversely affected by the policies, decisions, actions and inaction. They must ensure that JUSTICE IS SERVED and those responsible for these adverse health consequences and deaths. NO DEALS, JUST JUSTICE.
There are others also calling for recall. https://worldcouncilforhealth.org/resources/covid-19-vaccine-pharmacovigilance-report/