FOI to the Australian TGA Reveals Minutes of Covid-19 Vaccine Approval Meetings. Despite Unknowns, Despite Accumulating Evidence of Harm, TGA Proceeded to Approval All Doses to All Populations.
Minutes from 9 meetings of the ACV here. All endorsed covid-19 vaccination of the presented proposed groups, irrespective of the dearth of supporting information and the mounting evidence of harm.
Michelle Stubbs, citizen researcher, has been tracking reports of injuries from the Australian TGA since the rollout of Covid-19 Vaccines. Today she released a new report (on FB) presenting and compiling information obtained under FOI request from minutes of meetings held by the Australian Therapeutic Goods Administration’s (TGA) Advisory Committee on Vaccines (ACV) to review and approve the use of the Covid-19 vaccines for the Australian public. Michelle’s report is lengthy, links and screenshots are provided.
We do not know how long Michelle’s critical information will stay up on FaceBook. This information needs wide distribution. Thus, I have copied and am sharing in full below with only some formatting rearrangement, and with permission from Michelle.
This is evidence! Please save and share!
Michelle comments: -
What is most evident in all of the provided documentation is that no matter how much data/information is missing, the ACV always says YES!
There is substantial information contained in this FOI release. I certainly have not covered all of it, just what jumped out at me.
It continues to astound me that the ‘experts,’ who would be fully aware of the unprecedented number of reports of harm and death in the post market safety surveillance data of the covid shots, continue to refer to that data in a positive light.
I am yet to see a single expert even acknowledge the unprecedented number of reports of harm and death against the covid shots.
As always with the TGA, one must also consider the information that is not made available.
I note with concern that the ACV minutes concerning pregnant women receiving a Pfizer covid shot are not included in this FOI release despite being within the timeframe of the FOI request.
There has been a new release of information on the TGAs FOI Disclosure Log...The released documents detail the TGAs ACV – Advisory Committee on Vaccines1, meeting minutes between January 2021 and October 2022, but only on matters relating to the Pfizer covid shot...
1. 19 January 2021 ACV Meeting (Granted Provisional Approval of Covid-19 Vaccines for Persons Aged 16 and Over)
https://www.tga.gov.au/.../files/2023-03/foi-4093-01.pdf
This meeting was seeking ACV support for the provisional approval of the Pfizer Covid-19 shot for people aged 16 and over.
Unknown by the ACV at the time of this meeting: -
Protection from long-term complications secondary to SARS-CoV-2 infection, and effect on mortality, are not known.
Lack of data in children under 12 years (and limited data for those 12-15 years which was not reviewed in this submission), pregnant women, and lactating mothers.
No data on concurrent administration with other vaccines.
No data on mixed schedules with other potential COVID-19 vaccines.
An immunological correlate of protection is not established.
No data are available in the Aboriginal and Torres Strait Islander population.
The sponsor had not provided immunogenicity subgroup analysis by subdividing the age groups, such as 75-85 year olds.
There were few participants with renal failure as a co-morbidity, or other groups who may respond poorly to vaccines.
There are limited data to inform the duration of protection provided by this vaccine.
No studies of complement activation and cytokine stimulation.
Safety concerns to be addressed in ongoing and planned pharmacovigilance activities:
Anaphylaxis.
Vaccine-associated enhanced disease (VAED) including vaccine-associated enhanced respiratory disease (VAERD).
Use in patients with co-morbidities, including frail patients with co-morbidities (e.g., chronic obstructive pulmonary disease, diabetes, chronic neurological disease, cardiovascular disorders).
Use in immunocompromised patients, and patients with autoimmune or inflammatory disorders.
Use in pregnancy and while breast feeding.
Interaction with other vaccines.
Long term safety data.
There is limited or no information regarding patients with autoimmune or inflammatory disorders, immunocompromised individuals, pregnant women and individuals with a history of anaphylaxis. Clinical guidance will be required to assist individuals with decision making.
‘Guidance’ is required with decision making in the absence of information!
The ACV advised that it is unlikely for vaccine-related adverse events to occur more than 2 months after vaccination based on available data. However, there is limited information on the use of mRNA vaccine in humans, which underpins the need for post market vaccine safety surveillance.
Despite all of the above, the ACV supported provisional approval of the Pfizer covid shot for people aged over 16.
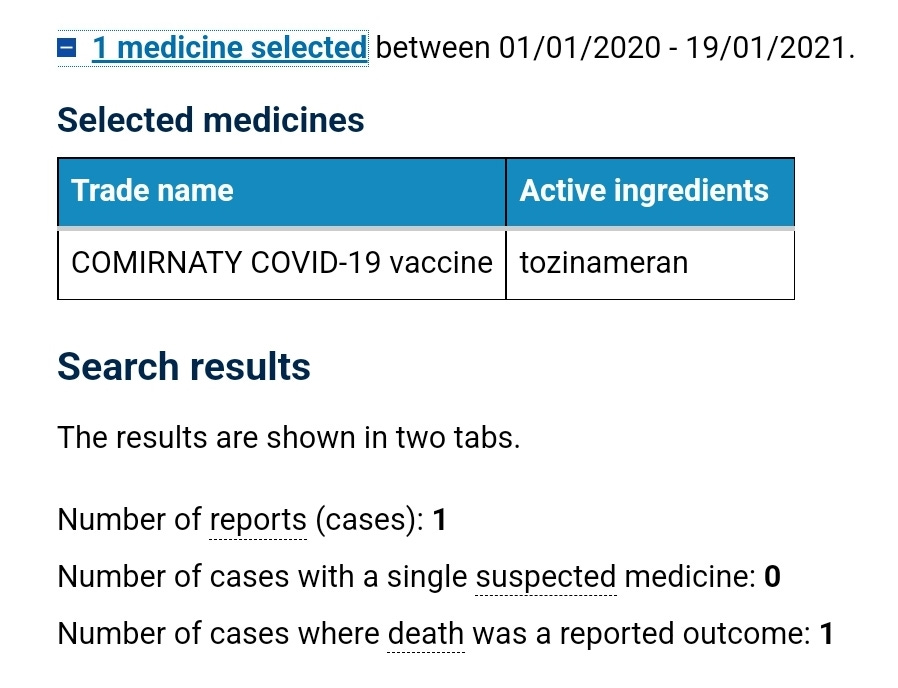
Below are the DAEN data as of 19 January 2021.
2. 16 July 2021 ACV meeting, adjourned to 5 July 2021 (Granted Provisional Registration for Individuals 12 - 15 Years of Age)
https://www.tga.gov.au/.../files/2023-03/foi-4093-02.pdf
This meeting was held to seek ACV support for the extension of provisional registration to individuals 12 to 15 years of age.
What wasn’t known by the ACV at the time of this meeting: -
The long-term efficacy and safety.
The vaccine efficacy against asymptomatic infection and viral transmission.
The number of adolescents in the reported study was not sufficient to detect rare adverse events.
No data on the co-administration with quadrivalent seasonal influenza vaccine.
Adolescents with immunodeficient status/high health risks are not specifically assessed.
The vaccine efficacy against variants of concern has not been assessed.
On myocarditis/pericarditis:
Rare cases of myocarditis/pericarditis following vaccination with Comirnaty have been observed in the global post-market setting.
Israeli analysis concluded there is a probably causal link between second mRNA COVID-19 vaccination and onset of myocarditis in males aged 16-30 years, with the highest risk in those aged 16-19 years.
Long term follow-up is important as problems (arrhythmias due to scarring of myocardium) may develop later in life.
Risks of the vaccine in the 12-15 years age group include increase risks in myocarditis and/or pericarditis and lymphandenopathy.
Information on longer term outcomes or recurrences is not available.
Despite all of the above, the ACV supported an extension of the provisional approval of the Pfizer covid shot to individuals 12 – 15 years of age.
At the time of this meeting there were 9,408 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 147 of those reports had an outcome of death.
This was already an unprecedented number of reports of harm as pre-covid the TGA received about 4,500 reports a year against all other vaccines, and with 5 or fewer of those reports having an outcome of death.
No mention of this alarming number of reports of harm was noted in the minutes.
3. 26 October 2021 ACV Meeting (Approved Provisional Registration of Booster Dose for Persons over 18 Years)
https://www.tga.gov.au/.../files/2023-03/foi-4093-03.pdf
This meeting was seeking ACV support for the extension of provisional registration to include a booster dose for those aged over 18 years of age.
The clinical data submitted related to 322 trial participants who were administered a booster dose. With only 12 of those participants in the 65 to 85 years age group.
The ACV noted the small numbers in the safety population (n = 306), predominantly aged 18 to 55 years. Rare but significant adverse events such as pericarditis / myocarditis were not observed but the power to detect these was very limited.
The ACV suggested the following changes to the Comirnaty PI:
Myocarditis and pericarditis
‘Very rare cases of myocarditis and pericarditis have been observed following vaccination with COMIRNATY. These cases have primarily occurred within 14 days following vaccination, more often after the second vaccination, and more often in younger men.’
Change ‘men’ to ‘males’, to use more inclusive wording for the younger age group.
‘The duration of protection afforded by COMIRNATY is unknown as it is still being determined by ongoing clinical trials and observational studies.’
The ACV supported the use of a Pfizer covid shot as a booster for those aged 18 and over.
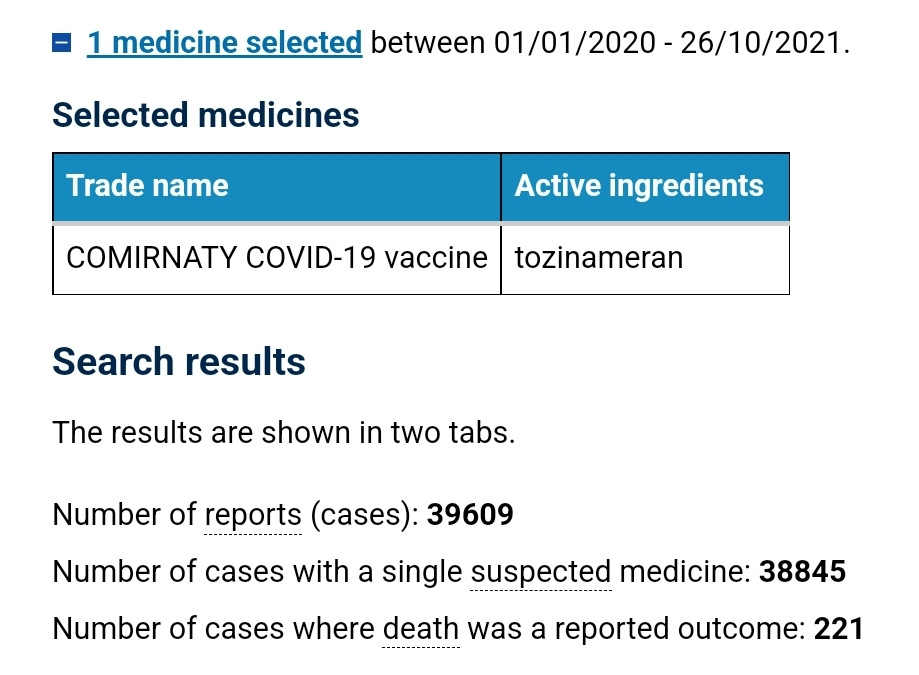
At the time of this meeting there were 39,609 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 221 of those reports had an outcome of death. Screenshot below.
No mention of this alarming number of reports of harm was noted in the minutes.
4. ACV meeting dated 6 December 2021 (Approved Provisional Registration for Children Aged 5 - 12)
https://www.tga.gov.au/.../files/2023-03/foi-4093-04.pdf
This meeting was seeking ACV support for the extension of provisional registration to include children aged 5 - 12 years of age. The sponsor is also proposing to use a new formulation (using TRIS2 buffer, replacing phosphate buffered saline [PBS] buffer) for Comirnaty in the proposed age group. However, the pivotal study was conducted using the old formulation (PBS).
THE PIVOTAL STUDY WAS A DIFFERENT FORMULATION THAN THAT BEING REQUESTED FOR APPROVAL!
The sponsor has proposed that the new formulation will subsequently replace the currently available formulation in Australia for all age groups. This has resulted in extensive changes in the product information, including storage and dilution requirements. This can lead to significant confusion, especially if the current vaccine stock (PBS buffer formulation) is also being used simultaneously.
This is what wasn’t known by the ACV at the time of this meeting:
Safety follow up is currently limited to median 2.4 months post Dose 2 in cohort 1 and 2.4 weeks for the safety expansion cohort.
Safety sample size is small.
The duration of immune response and vaccine protection is not currently known in the proposed age group.
Vaccine efficacy against asymptomatic infection and viral transmission are not known for the proposed age group.
The data in immunocompromised individuals are lacking.
Efficacy against the currently circulating variants of concern is not known yet.
The ACV discussed the epidemiology of COVID-19 in children aged 5 years and over.
The committee understood that to 10 October2021 no child under 11 years of age had died of COVID-19 in Australia. One child under 10 years of age has died following a COVID-19 diagnosis but it is understood that COVID-19 was not the cause of death. Children in Australia with COVID-19 rarely required ICU care (<0.1%).
The sponsor has also proposed two other variations within the same application:
Major variation [change in strength and dosage], to present 2 new strengths with different fill volumes, to support vaccination of different age groups with dosages of either 30 μg (≥ 12 years of age) or 10 μg (5-11 years of age)
Change in formulation, to change from a PBS/sucrose buffered formulation to a Tris/sucrose buffered formulation that can be used in all approved age groups.
The ACV noted that the absence of a controlled clinical study using the Tris/sucrose formulation proposed for provisional registration may contribute to vaccine hesitancy.
The ACV did not express any significant concerns with the lack of clinical data being provided to compare the two formulations. The ACV noted the positive benefits of the Tris/sucrose buffer formulation in relation to the extended fridge shelf life. The ACV noted that Tris buffers are widely used in other vaccines and this change in the buffer is not expected to have any safety implications; this may require specific communication to consumers.
We, the public, were informed about the changed requirements regarding refrigeration of the Pfizer covid shot, but were we informed that this was as a result of a changed vaccine formulation? What implications does this have in relation to all of the clinical trials using the original formulation that were relied upon in the registration process?
The ACV noted that all reported serious adverse events were deemed unrelated to the vaccine, while noting that the study was not powered to detect rare events.
The ACV noted that in the phase 1 study the observed reactogenicity in the initial 4/16 participants assigned the 30 μg dose (the adult dose) led to the discontinuation of this dose level. There is a clear risk of adverse events from administration of the adult dose in error to a child. Under the clinical trial conditions, dosing errors occurred in 3% of doses administered.
Immunogenicity against the Delta strain was considered by the ACV, and although slightly diminished compared to the original strain, there still appeared to be significant neutralisation antibody activity within this small subset of 34 participants. Vaccine-induced immunity against new variants, such as the recently reported Omicron variant, is not yet known.
The ACV supported the use of a Pfizer covid shot for those aged 5 – 12 years of age.
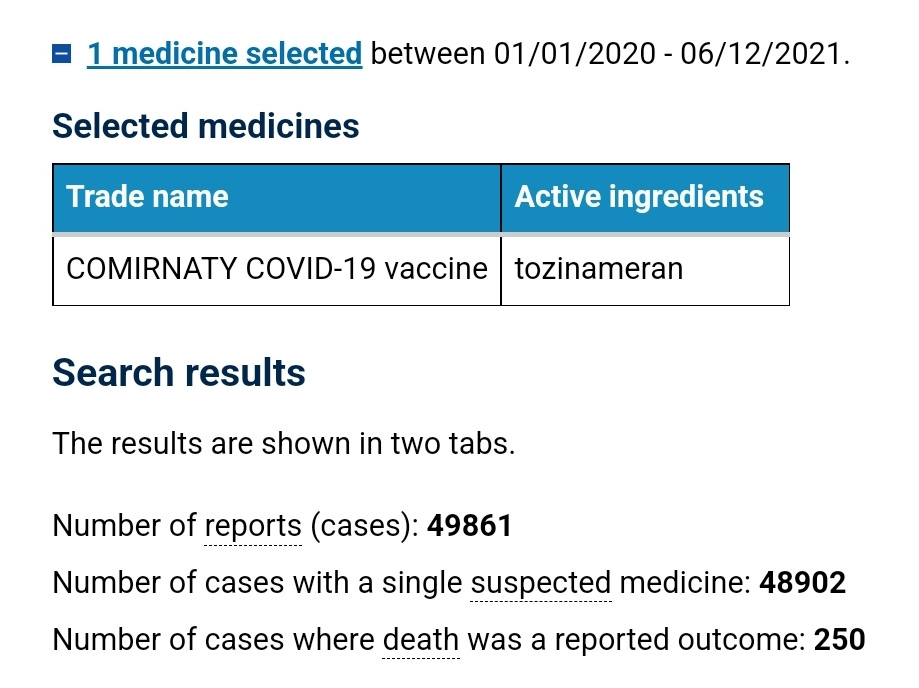
At the time of this meeting there were 49,861 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 250 of those reports had an outcome of death. Screenshot below.
No mention of this alarming number of reports of harm was noted in the minutes.
5. ACV meeting dated 14 January 2022 (Approved Provisional Registration of Booster Doses for Persons Aged 16 - 17)
https://www.tga.gov.au/.../files/2023-03/foi-4093-05.pdf
This meeting was seeking ACV support for the extension of provisional registration to include a booster dose for those aged 16-17 years.
The ACV noted the C4591031 study included 90 subjects aged 16 and 17 years within the study of 10,136 subjects aged 18 years and over.
The ACV noted that immunogenicity associated with a third dose against the Omicron (B.1.1.529) variant of interest was not available.
The ACV supported the use of a Pfizer covid shot as a booster for those aged 16 –17 years of age.
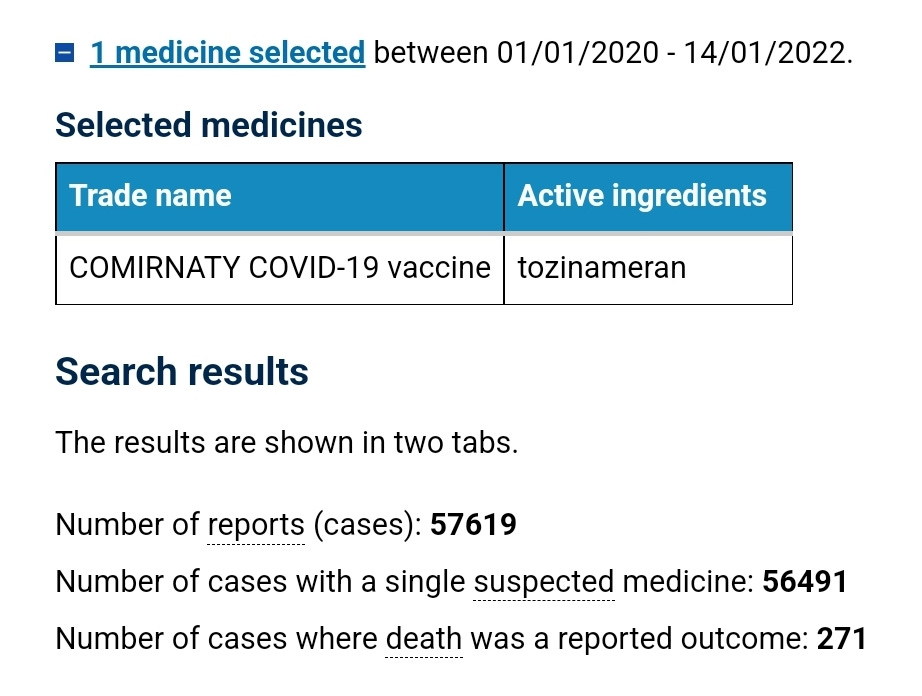
At the time of this meeting there were 57,619 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 271 of those reports had an outcome of death.
No mention of this alarming number of reports of harm was noted in the minutes.
6. 22 March 2022 ACV Meeting (Approved Provisional Registration of Booster Doses for Children Aged 12 - 15).
https://www.tga.gov.au/.../files/2023-03/foi-4093-06.pdf
This meeting was seeking ACV support for the extension of provisional registration to include a booster dose for those aged 12-15 years.
The delegate claims that ‘Safety is supported by real world surveillance of adverse events from Israel and USA.’
The ACV noted the application submitted by the sponsor on 22 February 2022 contained very limited data, all generated by regulatory authorities rather than the sponsor.
The ACV noted that no immunogenicity data had been provided from children aged 12-15 years.
Additionally, the submitted data only reported on less than 7000 children in this age group who had received a third dose, such that rare but significant events would not be expected to be detected in this number of children.
The ACV expressed reservations about the lack of data, a likely very small benefit against severe disease, and limited safety data from the USA and Israel.
The ACV agreed with the product information changes proposed by the sponsor, but proposed additional changes, including - articulate that there are key gaps in the available data and there is no immunogenicity data in this age group.
The ACV advised that rigorous post-marketing monitoring is required. It should be noted that there is no rigorous post market safety surveillance in Australia.
The ACV advised that updated data from the Israel and US CDC should be obtained and considered, given the minimal data provided by the sponsor.
Despite all of the above, the ACV supported the use of a Pfizer covid shot as a booster for those aged 12–15 years.
At the time of this meeting there were 70,751 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 316 of those reports had an outcome of death. Screenshot below.
No mention of this alarming number of reports of harm was noted in the minutes.
7. 7 September 2022 ACV Meeting (Approved Provisional Registration for Booster for Children Aged 5 - 12 Years)
https://www.tga.gov.au/.../files/2023-03/foi-4093-07.pdf
This meeting was seeking ACV support for the extension of provisional registration to include a booster dose for those aged 5-12 years.
The ACV noted that the clinical presentation of SARS-CoV-2 infection in children is usually mild, with many asymptomatic. The ACV further noted the low risk of severe disease with hospitalization within this population, with children with underlying medical conditions at a greater risk.
The ACV noted that Phase 2/3 study C4591007, which only allowed healthy pediatric participants, commenced on 7 June 2021 and is ongoing.
Safety and immunogenicity data are currently available for up to 1 month after a booster (third) dose. The immunogenicity data set comprised of up to 130 participants who received Dose 3 (3-Dose set) and up to 70 additional participants who received Dose 2 (2-Dose set).
The median follow-up time after Dose 3 was 1.3 months.
The clinical relevance of booster doses for this age group is unclear but may become important in the event of emergence of more transmissible/severe variants.
The ACV agreed that the demonstrated immunogenicity supports use in the population aged 5-11 years, although noted that an immunological correlate of protection is yet to be well defined.
The ACV acknowledged the limited data set, particularly for participants without prior evidence of COVID-19.
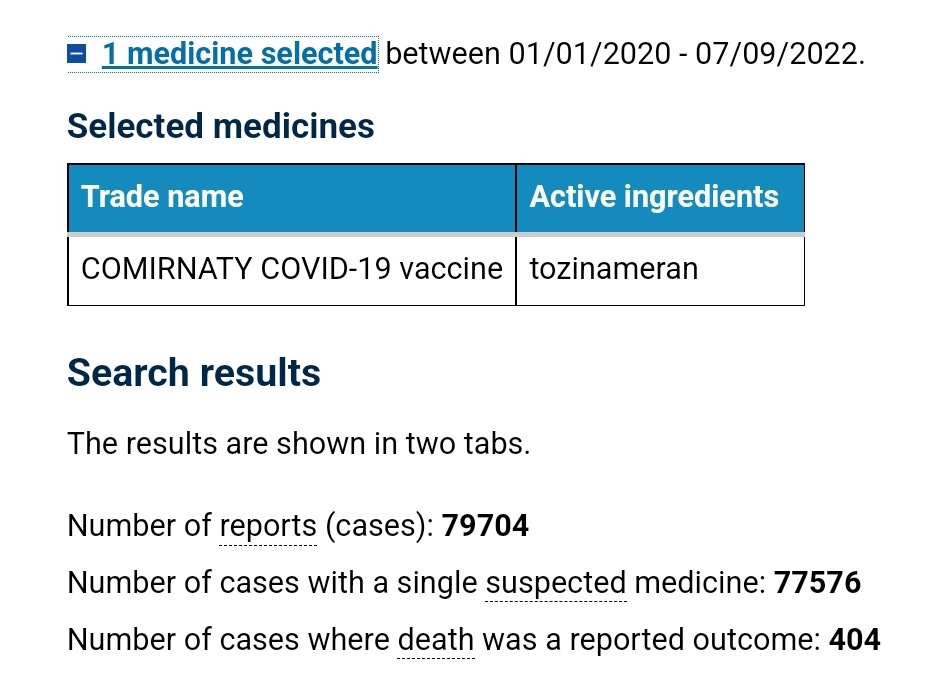
The ACV supported the use of a Pfizer covid shot as a booster for those aged 5–12 years, based on limited immunogenicity and safety data.
At the time of this meeting there were 79,704 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 404 of those reports had an outcome of death.
No mention of this alarming number of reports of harm was noted in the minutes.
8. 7 September 2022 ACV Meeting (Approved Extension of Registration for Children Aged 6 Months - 5 years)
https://www.tga.gov.au/.../files/2023-03/foi-4093-08.pdf
This meeting was seeking ACV support for the extension of provisional registration to include those aged 6 months-5 years.
The study C4591007 has only short term follow up (1.3 months following dose 3 in children 6-months to 23-months of age).
Neutralisation titre analyses were against ancestral strain, not the Omicron variant.
No vaccine-related events of anaphylaxis occurred. There were more cases of lymphadenopathy following Comirnaty than placebo, but the number of cases was small. One infant aged 6-months was reported to have a serious adverse event (eye rolling upwards) that occurred 2 days after Dose 2; febrile seizure related to vaccination cannot be definitively excluded based on available information.
The trial size was too small to provide data on rare outcomes post vaccination, such as myocarditis.
The ACV noted the absence of co-administration studies with influenza vaccine or the standard early childhood vaccines.
The ACV noted with concern the VAERS data (as of 21 August 2022; 496 reports) on Comirnaty vaccination in children 6-months to 4 years of age showing incorrect dose administered (18% of reports), product administered to patient of inappropriate age (11%), product preparation issues (10%) and wrong product administered (7%). VAERS data (as of 21 August 2022; 521 reports) on Spikevax vaccination in children 6-months to 5 years of age show expired product administered (7% of reports) and incorrect dose administered (7%).
The ACV noted that the study was not powered to detect rare events such as myocarditis and was supportive of robust post-marketing safety monitoring. I’m just pointing out again that ‘robust’ post market safety surveillance is not available in Australia and the ACV would be very aware of this.
The ACV highlighted the potential for administration of a COVID-19 vaccine to disrupt the well-established early childhood vaccination schedules for other childhood diseases. In the absence of co-administration trials, studies monitoring potential interactions should be undertaken.
The ACV supported the use of a Pfizer covid shot for those aged 6 months–15 years, on the basis of short-term efficacy and safety data.
At the time of this meeting there were 79,704 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 404 of those reports had an outcome of death.
No mention of this alarming number of reports of harm was noted in the minutes.
9. 5 October 2022 ACV Meeting (Approved Extension of Provisional Registration for Bivalent Booster for Persons Over 18 Years of Age)
https://www.tga.gov.au/.../files/2023-03/foi-4093-09.pdf
This meeting was seeking ACV support for the extension of provisional registration to include the bivalent booster for those aged over 18 years of age.
This is what wasn’t known by the ACV at the time of this meeting:
No data for the bivalent vaccine in individuals under 55 years of age.
No data at all for the 12 years to 18 years population.
No data for the bivalent vaccine following 2nd and 4th doses of Comirnaty.
Immunogenicity against the currently circulating subvariant (BA.4/5) is not known.
Safety sample size was small and follow up was limited to 28 days post booster dose.
Immunogenicity follow up duration is short, and the long-term trend/duration of immune response post booster dose is unknown.
Clinical efficacy and efficacy against asymptomatic infection and viral transmission were not studied.
No data available for immunocompromised individuals, uncontrolled co-morbidities and frail elderly.
No data available in pregnant women and lactating mothers.
Also, there are no data whatsoever for 12-17 years age group. The benefit risk balance in this age group cannot be decided in the absence of data, particularly where there are very limited data in younger adults. Adolescents and young adults are at highest risk of the most important known safety risks following mRNA COVID-19 vaccines.
Safety sample size in Substudy E for the proposed formulation was small (n=305).
Follow up was limited to 28 days post booster dose. At the data cut-off, only 10.4% of participants had ≥ 2 months follow-up.
The ACV noted that a purpose of variant COVID-19 vaccines in general is to increase the breadth and depth of immunity rather than to keep pace with the evolution of SARS-CoV-2 (as this is impossible). The role of an Omicron-specific booster is unclear.
The Interim Clinical Study Report (1-month analysis) for the pivotal Substudy E included only participants aged over 55 years. There are no efficacy endpoints.
The ACV supported the use of a Pfizer bivalent shot for those aged 18 years and over, on the basis of immunogenicity and short-term safety data.
At the time of this meeting there were 79,978 suspected adverse reaction reports on the TGAs DAEN against the Pfizer covid shot, and 406 of those reports had an outcome of death.
No mention of this alarming number of reports of harm was noted in the minutes.
If you feel so inclined, you can support Michelle’s work by shouting her a coffee via this link... She states “the information I share will always be free and gifts of coffee are not mandatory...”.
Thank you for your dedication, Michelle! Truth is going to win!
Link to ACV details.






Thank you for republishing this important information Sally.
What it tells me is that the government-appointed bureaucrats who headed regulatory agencies such as the TGA, ATAGI, etc., were unsuited to hold those positions in what was, in effect, an emergency situation.
Individuals in bureaucratic organisations - whether public or private – often gravitate to the top management roles over many years due to factors unrelated to their personal competence or ability to manage a crisis. For example, an individual might be able to secure ongoing promotion up through the ranks of an organisation because he/she is astute at office politics, or simply serves the time. However, when a full-blown crisis arises with multiple compounding factors that may be beyond the life experience of a bureaucratic officeholder, suddenly you have the wrong person in the wrong job. Disaster then follows.
Historically, this syndrome often manifests in wartime situations when it becomes apparent that a peacetime appointee (say, an army general) cannot do the job, and survival of the nation may be put at risk. In the lead-up to World War II, the Australian Government of the day gave the Commonwealth Defence Department the job of re-arming the country to prepare for coming war. The bureaucracy dithered and proved incapable of doing the job. Important decisions got lost in a maze of interdepartmental committees. In the end the Menzies Government brought in an outsider - the head of a large manufacturing/engineering conglomerate and appointed him the supremo of defence support. It worked, he cut through the bureaucratic blockages and solved the problem. War materiel production finally got under way. By the end of WW2, Australia had effectively transformed into an industrialised country.
The moral of the story is that in times of crisis, the urgency of the situation often requires the previous bureaucratic appointee’s immediate replacement with someone who has the intellectual and organisational capacity to take over command and get results. This clearly did not happen in Australia when the Covid crisis hit. The Government ministers responsible simply left the ‘peacetime’ limited perspective bureaucrats in place, firmly embedded in their group-think feedback loops, not understanding that their primary responsibility was to protect the public – in this case from commercially rapacious pharmaceutical corporations.
As the Covid crisis deepened, both the Australian Federal Government and the various State Governments collectively failed to execute their responsibility to the public. They did not ensure that capable people were in place, or management structures established, to take oversight command of the crisis away from the bureaucratic drones. It was absolutely unforgivable that the TGA and its advisors - from early in the crisis - deliberately removed the availability of historically-proven early treatment options for Covid, and then used the media to facilitate the crushing of any public dissent – even when raised in Parliament. The consequent demonisation and then removal of former MP Craig Kelly from parliament is a stain on the political history of this country, and the former Prime Minister needs to beg forgiveness for doing that. The banning of the early treatment options was clearly done to force the use of the mRNA injectables as the only ‘treatment’. That despicable act alone sent many Australians an early death.
At the end of the day, the captain of a ship always has to accept responsibility for putting his ship up on the rocks. In Australia’s case, I would attribute the ultimate blame to the various ministers for Health - Federal and State - plus the Prime Minister and State Premiers. These were people who had the constitutional power, but were too busy playing party politics to carry out the research and intellectually equip themselves with the necessary understanding of what their responsibilities were in the growing crisis. A key responsibility was to ensure that the governmental bureaucrats could do the job for which they were employed.
The direct result of this political incompetence is hundreds of thousands of permanently ‘vaccine’-injured Australians as a result of the inadequately-tested novel mRNA genetic injectables, plus tens of thousands of unnecessary premature deaths, and many years of future deaths, not to mention the tragedy of the stillborn. The people responsible for this failure of governmental management need to be exposed and held accountable, or it will simply happen all over again.
Once again, Sally thank you for publishing this information, and thank you Michelle for fighting so hard to obtain it. It reveals the incompetence of the bureaucrats who run the TGA and their various advisors. My hope for the future is that all these people will be subjected to a public inquiry so that they can be legally forced to explain their actions to the injured and the bereaved.
Criminal acts world over. Nobody getting "IT" yet? Acting in unison? Lockstep?
https://andybunting.substack.com/p/awake