How to Report a Suspected Adverse Reaction Following Covid-19 Vaccination in the Philippines
It took me a while to find out how to make a report. Sharing typical reactions and reporting resources here.
Reporting of adverse events following immunization (AEFI) is critical to enable safety monitoring of the 9 covid-19 vaccines that are currently approved under Emergency Use Authorization (EUA) in the Philippines.
There was no link that I could find from the DOH Covid-19 Vaccination possible side effects page to the Philippines reporting system (under DOH-FDA). Mostly mild effects are acknowledged on the DOH page. Severe reactions are stated to be rare.
AEFI can affect many body systems, may be short lasting and relatively mild, can be acute and sudden, even deadly, or can develop more slowly over time in months or even years. Some effects may only become apparent in the offspring of recipients (epigenetic effects) and may take generations to become apparent.
Ideally any new condition developing after vaccination is reported, even if is not known if it is linked, because population health data is critical in detecting signals of adverse reactions.
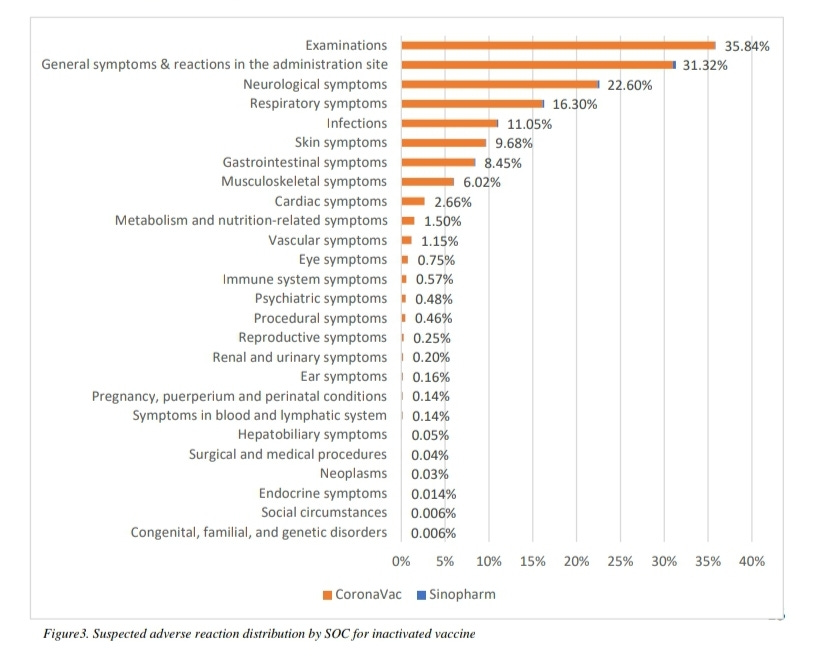
Adverse reactions being reported in the Philippines vary by vaccine type, but with top adverse reactions including neurological, gastrointestinal, respiratory, cardiac, immunological, and reproductive. Currently there are also increasing reports of cancers. The latest available 10th April 2022 Pharmacovigilance report is linked. It refers to 97,440 AEFI reports including 2,138 reports of deaths, though it also points out that reported AEFIs are not necessarily linked to the received vaccinations.
For easy reference screenshots of the most common adverse reactions by vaccine types extracted from this report are shown below.
A link to the FDA reporting system is provided here. You will land on the page shown below, which will allow electronic submission of a report.
Following submission, you may be contacted by the pharmacovigilance team to send them further information such as hospital abstracts, death certificates, etc. You will also be assigned a case number.
I went through this process for a previously healthy person who died of a sudden and catastrophic cardiac event within 48 hours post 2nd dose of a covid-19 vaccine late last year. I didn’t hear anything back after submission of the requested additional information, so I cannot comment further on the standard procedures.
If you or anyone you know experiences any possible AEFI, please make a report! Our vigilance on this can make a difference.





Thank you for writing this! Earlier this year I was curious about the site and went to visit, it used to be hidden in a difficult-to-find link in the FDA website. I'm sorry that I was not able to keep the screenshot I took back then, it would have been an interesting basis for comparison.