HPV Vaccines in Philippines. PHP2 billion spent on procurement from 2022 to 2024. What is HPV? WHO Initiative for Cervical Cancer Elimination. Vaccine Insert. Safety Concerns & Resources!
The WHO is driving HPV Vaccination of young people for elimination of cervical cancer. However, there are safety and efficacy concerns with these vaccines.
PHP2 Billion for HPV Vaccines from 2022 to 2024
The Philippines Government DOH allocated PHP2 Billion to procure HPV vaccines for the population from 2022 to 2024. While the key target is 9-year-old girls (before they become sexually active / are exposed to HPV), older girls and women who have never received this vaccine are also being targeted for up to 3 doses of this vaccine.
Congressman Dan, who had been conducting the hearing into excess deaths over the pandemic, is offering this vaccine “free” to his female constituents.
What is HPV?
HPV is Human Papiloma Virus. There are more than 200 known strains of HPV, some of which are associated with oral, throat, genital, and anal cancers and warts. Medical researchers developed vaccines targetting prevention of HPV infection, optimistic that this would also prevent associated cancers.
HPV Vaccines & WHO Cancer Elimination Iniative
HPV Vaccines first became available in 2006. There are 2 brands of HPV Vaccines: Cervarix which is said to prevent cervical cancer arising from HPV types 16 and 18, and Gardisil. Gardisil has 2 formulations; Gardisil-4 said to protect against HPV types 6, 11, 16, and 18, and Gardisil-9 (introduced in December 2014) said to protect against HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58 (types 6 and 11 are associated with genital, anal, and oral warts).
The WHO is pushing HPV vaccines with the objective of eliminating HPV-related cancers by 2080, article.
Vaccine Insert for Gardisil-4
Assuming that the product offered for the school program in the Philippines is the cheaper Gardisil-4, the prescribing information can be reviewed on the product insert (click below).
The vaccine is recommended for girls and women, boys and men aged 9 to 26 years of age. It is also emphasized that the vaccine must be given before exposure to HPV; if given after exposure malignancies may be accelerated.
It should be noted, from the vaccine insert limitations section, that the vaccine does not eliminate the need for screening, does not provide protection if the recipient has already been exposed to HPV prior to the vaccine, may not protect all recipients, and has not been demonstrated protective in women older than 26.
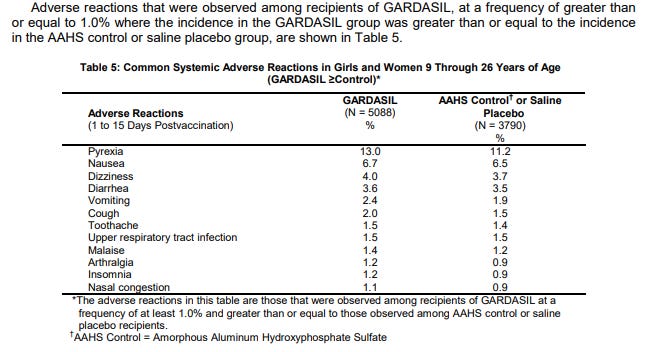
The studies for the vaccine used the vaccine (N=5088), a saline placebo (N=320) and the vaccine AAHS aluminum adjuvant as a control group (N=3470). The report only looked at the Saline placebo in the injection site adverse reactions. Clearly the full product had more reactions than the AAHS Control than the saline.
However, when it came to serious reactions, they combined the saline and AAHS into one group. This is very disingenuous because the AAHS adjuvant is highly reactive and causes severe reactions in its own right. By hiding the results of the saline placebo and saying both the vaccine and the combined control/placebo groups had similar reactions, they could claim that both regimes were as safe (or as dangerous) as the other.
The insert only listed adverse reactions that occurred in 1% or more of recipients; meaning rare and serious events that occurred at a lower incidence than 1 in 5 for the Gardisil group could be hidden.
Both the Vaccine and the AHHS Control lumped with the Saline group appear to have high rates of new conditions post injection. These are serious conditions for young healthy women.
Cervical cancer is not typically a disease of girls or young women or girls; it is a disease of late middle age and older. The girls receiving these vaccines could not expect to see any benefit for decades. So how did the manufacturer determine that the vaccine prevents cancer? They couldn’t! That would take decades to determine. Instead, they used a proxy marker, of pre-cancerous lesions, which often resolve spontaneously with no treatment.
Gardisil-4 has not been evaluated for ability to cause cancer or genotoxicity. Reassurance for impacts on fertility was based on a rat study.
Post-marketing experience, Ie. after licensing adverse events are listed. These are very important, as the studies can be too short and too restricted to capture the scope and severity of adverse events.
Safety and Efficacy Concerns with the HPV Vaccines
There are major concerns that the HPV vaccines are not preventing and instead may be contributing towards cancers (type replacement for the targeted HPV types with more dangerous strains). These vaccines may enhance rather than reduce cancers.
Further there are major concerns with the safety of the HPV Vaccines. Selected resources are provided.
HPV Vaccines Did Not Use a Placebo
Page 11, of the linked document:
Conclusion
The Philippines is complying with WHO objectives to ensure all eligible children and young women receive HPV vaccines. The price tag is considerable at more than PHP2 billion just for 3 years procurement, even before distribution, handling and administration costs.
There are serious concerns that the HPV vaccines may not be delivering the reduction in cancer that was promised, and that adverse reactions can be very severe. Adverse reactions, in poor settings that cannot access adequate medical care, may have even worse outcomes than they would in developed nations where supportive care is more available.
HPV vaccines are given to young women decades before they would at risk of cervical cancer. If these young women (and men if given to boys to address risk of warts and anal cancers / to “protect their partners”) suffer from adverse reactions, including autoimmune and neurological, they have to deal with these for decades before any benefit might(?) be realized. This is a huge loss of QUALYs (quality life years) for the affected people.
Even with HPV vaccines, women must still undergo screening for cervical cancers. Thus, there are no savings for the health care system in this aspect.
There is some indication that cancer rates may be increasing among vaccinated girls. There are grassroots accounts of reduced fertility and premature ovarian failure related to HPV vaccination, although this is denied by most medical providers and the WHO. Teenage pregnancies have also been dropping globally. Is the global drop in fertility and birthrates related to HPV vaccination?
Parents of 9 year old girls, and young women (and men) who may take these vaccines need to understand that these vaccines have benefits and risks. Informed consent is required and should be resepected!
Could PHP2 Billion over 3 years be better spent on general health and wellness education, on cancer screening and treatments, and on other projects?
























Great article. I have a daughter who has been offered the HPV injections multiple times. This is very helpful. Thanks.
Q: IS VACCINATION MANDATORY?
A: No, vaccination is not mandatory. But the government highly encourages the public to get vaccinated and be protected against preventable disease.
https://caro.doh.gov.ph/episode-4-is-vaccination-mandatory/