Is the Philippines DOH Violating Its Own FDA EUA Conditions for COVID-19 Vaccines Use? Serious Questions on the Use of Heterologous Dosing, Timing of Boosters, and on Permissibility of 2nd Boosters!
Public domain EUA Approvals: 1st booster timing is 6 months. No Clause covering 2nd Boosters. No Heterologous Boosting except Janssen/Sputnik Light. Use outside EUA Scope? Booster Moratorium?
Last 9th August 2021, the National Covid-19 Vaccination Operations Center issued Advisory No. 72, advising against the administration of additional (booster) doses of Covid-19 Vaccines aside from those covered by the FDA EUAs. The advisory is provided in full below.
Then on September 6, 2022, there is an addendum, also included in the link above.
I excerpt to make it more legible:
The advisory provides a directive against the administration of additional (booster) COVID-19 doses aside from what is recommended under the Emergency Use Authorization for the FDA. Vaccine recipients shall only receive the recommended dose series from one (1) COVID-19 Vaccine Brand (English).
Am I reading correctly? This issues 1) a caution on boosting outside of the EUA terms, and 2) requires only homologous (single brand) dosing?
A closer look at vaccine dosing protocols and at the current EUA documents is required. I have focused on the Pfizer EUA, as the Pfizer vaccine is currently the most frequently used product in the Philippines (42% of overall doses, but 92.2% of the doses administered in October 2022).
Heterologous Covid-19 Products Dosing Widely Used in the Philippines
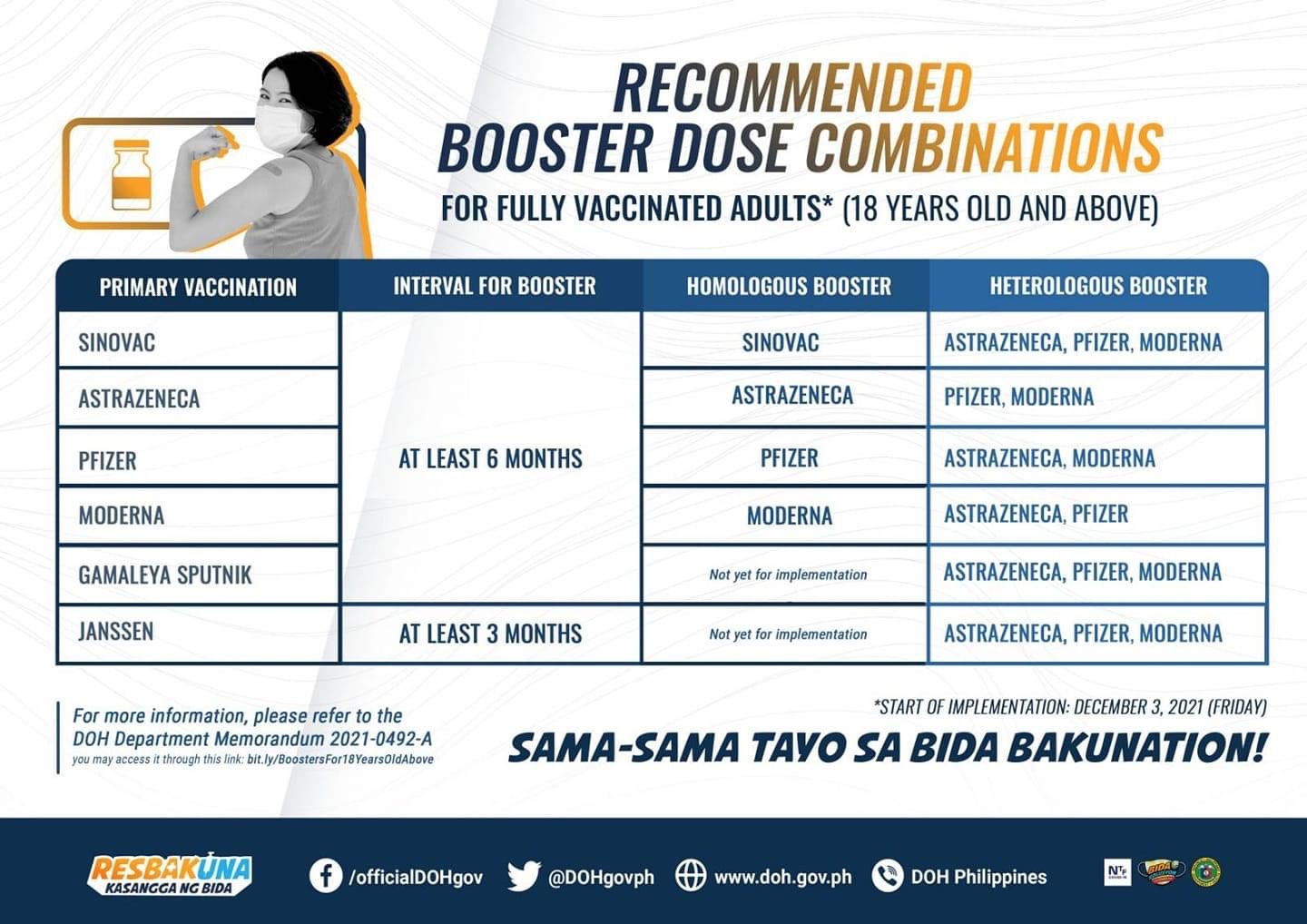
While the initial recommendation back in October 2021 had been for homologous boosting (stick with same brand for all doses), soon after, mix and match approaches (heterologous dosing) were promoted. People were advised to get whatever was available regardless of which doses they had previously received.
Then the DOH advised that the interval for boosting was reduced to 3 months for all brands, except Janssen which was set at 2 months.
People were also advised to get jabbed if they hadn’t already been and/or boosted immediately following a Covid-19 infection, provided they no longer had fever for 24-hours and respiratory symptoms had improved! I did hear of a case who had Covid-19 and was hospitalized and recovered. That person was then injected prior to leaving hospital, and subsequently died shortly thereafter. Blamed on Covid-19, of course!
That last sentence!
THANK YOU for trusting the vaccines that are SAFE, EFECTIVE, and FREE!
Pfizer products are currently provided as the main choice for booster products.
Many people have taken far more than the EUA recommended three (3) doses. I have personal friends who first got 2 doses of Coronavac (Chinese brand product), then they got 2 doses of Moderna for a second primary series, and now they have already received two booster doses of Pfizer. 6 doses! Where do they now stand?
I have even heard, first-hand accounts and people I know, of people who have taken more than 2 boosters of various products. Few questions are asked at vaccination centers, and a person can get as many doses as they want if they have several vaccination cards. Given the ubiquitous DOH ‘safe and effective’ messaging, I have heard of people, not perceiving any possible risk, who said that they will get (and they took) an additional dose any time there was a report of a new outbreak.
Last April 2022, the DOH requested the FDA for updating of EUA Documents to allow 2nd Booster Dosing for 12-17 yos.
I wanted to see if 2nd boosting is covered, and checked the EUA documents, all publicly available on the Philippines FDA Site. Unless there are other documents not publicly disclosed, the following observations on the EUA Products apply: -
COVID-19 Vaccine Pfizer EUA Documents
The stated pediatric vaccine is Comirnaty.
The latest pediatric (5-11 yo) EUA is dated 26 April 2022. The indication is stated as “for the prevention of Covid-19”.
Pediatric dosing covered by the EUA is for 2 doses. There is no mention of a 3rd dose.
The latest adolescent and adult (12 yo plus) Pfizer product EUA is dated 14 June 2022. The adult product is not Comirnaty, it is BioNTech.
This product is indicated for the “prevention of COVID-19”, and three (3) doses are authorized under this EUA, with the 3rd Dose being permitted 6 months after the second. Limited safety data is noted with reference to the third dose. There is no mention of a 4th dose (2nd booster). There is no mention of heterologous dosing.
Heterologous Boosters
I checked all of the EUA documents available on the FDA website for the “approved” Covid-19 Vaccines with regards to boosting indication. Sputnik and Sinopharm are each only approved to be given as a 2-dose primary series. Moderna, Pfizer, Coronavac and AstraZeneca are approved for 2 doses and a booster. Only Janssen and Sputnik Light are authorized to be given as heterologous boosters after different brand primary dosing. For Janssen, the booster doses are allowed in 18 yo and above, and the EUA states a two-month minimum interval for boosting. Sputnik Light may be given as a booster dose to persons 18 years and older 6 months after completion of another brand primary series.
If no other products specifically state that they are permitted to be given as heterologous dosing for boosters, then it follows that they are not approved to be used as such! Exactly, as stated in the first document above “Recipients shall only receive the recommended dose series from one (1) Covid-19 Vaccine brand”.
More Questions than Answers
Given all of the above, the following questions are now raised:
If it has been shown definitively that these products cannot deliver on their stated indication of “prevention of COVID-191” can these EUAs still be valid?
Can these Products be given to the population in a way that is outside of their FDA EUA authorization and scope?
No products currently have an authorization for 4th doses (2nd boosters). The maximum number of authorized doses are three for adults and children 12 years and older.
No products except Jansen and Sputnik Light have EUA authorization for heterologous use.
Is the DOH able to extrapolate and extend the Covid-19 vaccination to applications which are not specifically set out in the FDA EUA documents? Do they have this authority? The EUAs state that vaccination providers may only administer each product in accordance with its EUA?
Why were Filipinos permitted to receive more than one primary series of different vaccines? Many took primary series twice, with 2 different products. FOI request was submitted last 4th October 2022 asking if the DOH has been tracking information on people who have taken multiple doses, with no response received to date.
Why was the heterologous use of vaccines permitted and promoted, particularly for booster doses (Pfizer is currently the most used product), when this is not covered by the EUA documents?
Why is the gap between primary series and booster dose reduced to 3 months, when the EUA documents state a gap of 6 months (except Janssen, which allows 2 months), and have not been updated to allow a shorter interval?
If a recipient is injured or killed following the unauthorized use of these products, who is responsible and liable? The victim, or the person / center administering the product and/or the DOH?
If a person is injured or killed after taking a 2nd booster dose (approximately 3.5 million Filipinos to date), or an unapproved heterologous dose combination (unknown 10s of millions), will they (or their surviving family) still be eligible to apply for compensation / assistance under the Philhealth Vaccine Compensation fund?
Are the liability waivers signed by vaccine recipients valid if these products are used in a manner outside of their authorized use?
Has DOH Acted outside its jurisdiction? Even if EUAs were to be updated, that updating cannot be retrospective! What now?
Given all of the above, shouldn’t an immediate moratorium on all booster dosing already be called? Shouldn’t a senate enquiry be convened?
Philippines constitutional and medical ethics lawyers are called upon to comment!
"He has shown you, O mortal, what is good. And what does the Lord require of you? To act justly and to love mercy and to walk humbly with your God." (Micah 6:8)
Acknowledgment and Thank You
A special thanks to the lightworkers, legal team, and sounding board friends who had inputs on this post! You know who you are! I absolutely could not do this without you all!
Pfizer, via Ms Janine Small last 10 October 2022, has already stated during the EU Parliamentary enquiry that their products were not tested for prevention of transmission. Real world data has shown that Covid-19 cases escalated everywhere these products were rolled out. It is already an accepted fact that these products do not stop infection or transmission. The narrative was then moved to “they prevent serious infection”. Regardless, the documented EUA indication is already defaulted!















Thank you Supersally for this timely shout out! I pray for the children of the Philippines that people and legislators will finally come to the realization of what is happening here. The government must at the very least stop giving free shots as if they are for some kind of a lucky draw winners
I called it several months ago:
-Appointing a lawyer as DOH Undersecretary is to have him go through all the Department's directives and start closing up legal loopholes for people to sue them for, later on. This is a form of preventive "amnesty"--- in the provax perverted application of the word amnesty. Ie, "Yes, we made a mistake when we violated the EUA by telling people to get whatever is available, even if it's not homologous--- but look, we corrected ourselves so now you can't complain because we amnestied ourselves" :P
-Appointing an ex-PNP police chief as DOH Undersecretary is to have him go through securing the facilities of the DOH compound, plan for escape routes of employees during mob/mass action protests, and build and train an internal security force to quell protests.
They are circling the wagons. They know NOTHING CAN STOP WHAT IS COMING for them. This was never about health--- they were the arm of WEF for depopulating as many Filipinos as possible.
Vergeire started walking her statements back in a controlled, casual manner just a week or so after the lawyer was appointed DOH Usec.
#damagecontrol
#NoAmnesty
#Nuremberg 2.0