Philippines FDA Covid-19 pharmacovigilance reports conclude that there is no "basis for revising the current recommendations regarding the use of Covid-19 vaccinations"
AEFI reports (97,440), deaths (2,138), and Covid-19 breakthrough cases (4,425) are strongly positively correlated with vaccine doses (144,072,000); correlation coefficients range from 0.95 to 0.99.
RA11525 is “An Act Establishing the COVID-19 Vaccination Program Expediting the Vaccine Procurement and Administration Process, Providing Funds Therefor, and for Other Purposes” was signed on 26 March 2021.
Included in this act are definitions of Adverse Events (Item V. K.), the setting up of a Covid-19 Vaccine Information Management System (VIMS) (Item VI. H.), and the requirement for monitoring all vaccine recipients for Adverse Events Following Immunization (AEFI) and providing referral to appropriate facility for management (Item VI. I.). A Covid-19 National Vaccine Indemnity Fund was to be set up and administered under Philhealth as a trust fund to compensate any recipient who experienced severe adverse reaction.
The Inter-Agency Task Force for the Management of Emerging Infectious Disease (IATF-EID) were tasked to “establish a Special Task Group composed of medical and vaccine experts with proven track record, who will be in charge of monitoring the probable adverse effects following immunization from COVID-19. The Special task Group shall promulgate the necessary guidelines on the monitoring, evaluation investigation, and reporting mechanism to be followed by all LGUs and private entities.”
To date, I have not found any public domain publications relating specifically to this task or its delivery; if any reader has access to such information, please comment below.
The Philippines FDA has been conducting pharmacovigilance and issues weekly reports on “Reports of Suspected Adverse Reaction to Covid-19 Vaccines”. These reports define Serious Adverse Effects (SAEs) as Adverse Events Following Immunization (AEFI) that result in:-
In-patient hospitalization / prolongation of existing hospitalization
Significant disability / incapacity
Life-threatening (e.g. anaphylaxis) and death
Birth defect or congenital malformations
Considered to be medically important event.
We should note that the Philippines Pharmacovigilance system is likely subject to the same gross underreporting which is documented in international reporting systems, but we do not have data to define the magnitude of this under-reporting (perhaps 10 to >100x?).
Although vaccination of pregnant women started in October 2021, no adverse reactions for this group were reported until the 16th January 2022, and no indication if any of the serious AEFIs included death has been provided. Vaccination of adolescents began on 15 October 2021 and the first AEs appeared in the 31st October Report. Vaccination of the 5 - 11 age-group began on 7th of February 2022 with the first AEs reported on 13th February.
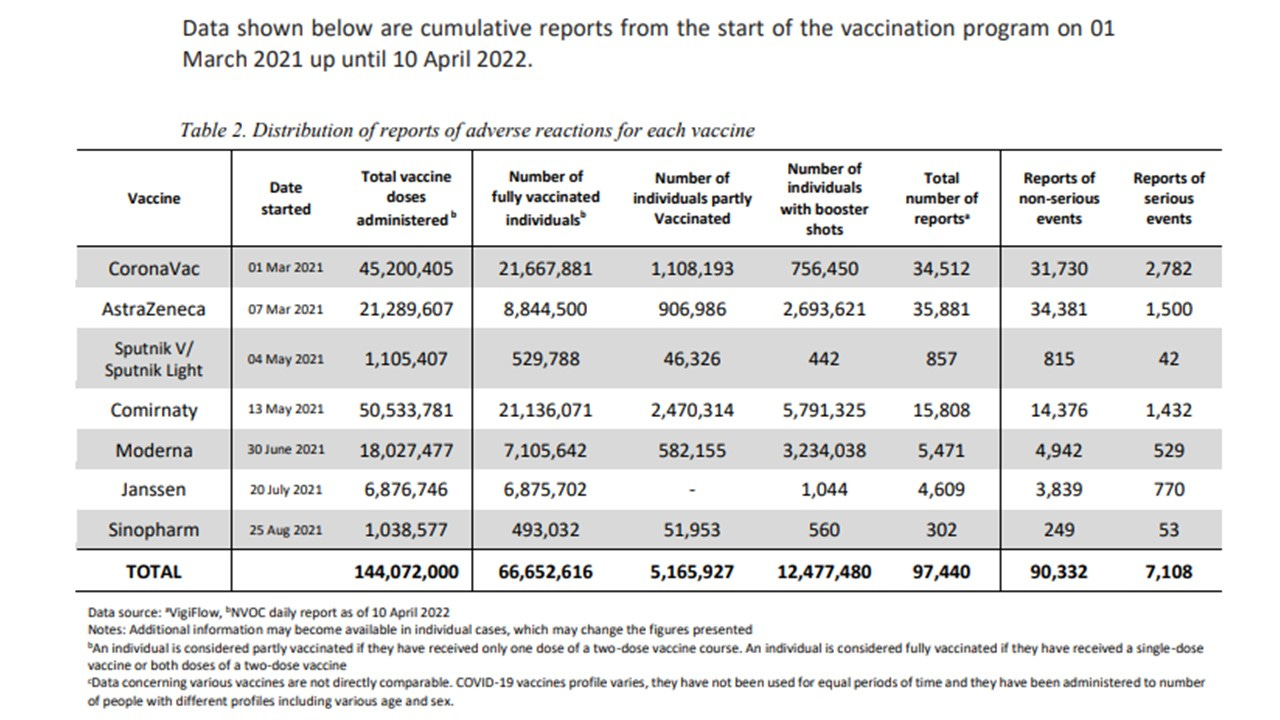
The latest available FDA report, issued on 10th April 2022, includes 90,332 reports of non-serious events and 7,108 reports of serious events which includes 2,138 fatal events (7 years and older).
The 2,138 total deaths reported are broken down by age.
The FDA report summary states “As per benefit-risk assessment, these reports do not provide a basis for revising the current recommendations regarding the use of COVID-19 vaccines”. They point out that most reports do not mean that the vaccine was causative and imply that cause could mostly be attributed to underlying conditions.
Correlation between Vaccine Doses and AEFIs
I have been tracking and summarizing data from the FDA reports. I wanted to see if there is an possible relationship between vaccine doses, total reported adverse events (mild and serious), total reported deaths, and total covid-19 infections.
To do this this, I plotted the weekly total vaccines against the weekly total reported AEFIs. I calculated Pearson Correlation Coefficient for the subject sets of data; 0 means no relationship, -1.0 means a perfect negative relationship, and +1.0 means a perfect positive relationship. All plotted data sets had a Pearson’s R of 0.95 to 0.99, indicating a very strong correlation. I.e. reports, adverse reactions, deaths, and breakthrough Covid-19 cases all increase synchronously as vaccine doses increase.
For those people who developed a confirmed COVID-19 infection following vaccination 5.13% died. This data may be skewed, as those who became seriously ill with covid-19 following vaccination may be more likely to report their experience than those who developed only a mild self-limiting illness following vaccination. However, this data clearly puts lie to the claim that the vaccines prevent serious illness and death from Covid-19.
The FDA report contents do not provide sufficient data further detailed analyses to be conducted. A detailed review of the Pharmacovigilance data by expert epidemiologists and medical personnel for cause and effect is recommended.
Please also see my previous article last 27th March 2022 regarding AEFI and determination of causation, which criteria I believe have been met.