Philippines FDA Pharmacovigilance Report from 30 May - 5th June 2022 adds 44 Additional Deaths Following C-19 Vaccination. When Will FDA Recall These EUA Products?
FDA claim No Signal despite their systems allowing Class Drug Recall based on reasonable suspicion of harm. World Council for Health New Report Calls for a Universal C-19 Vaccine Recall due to harm!
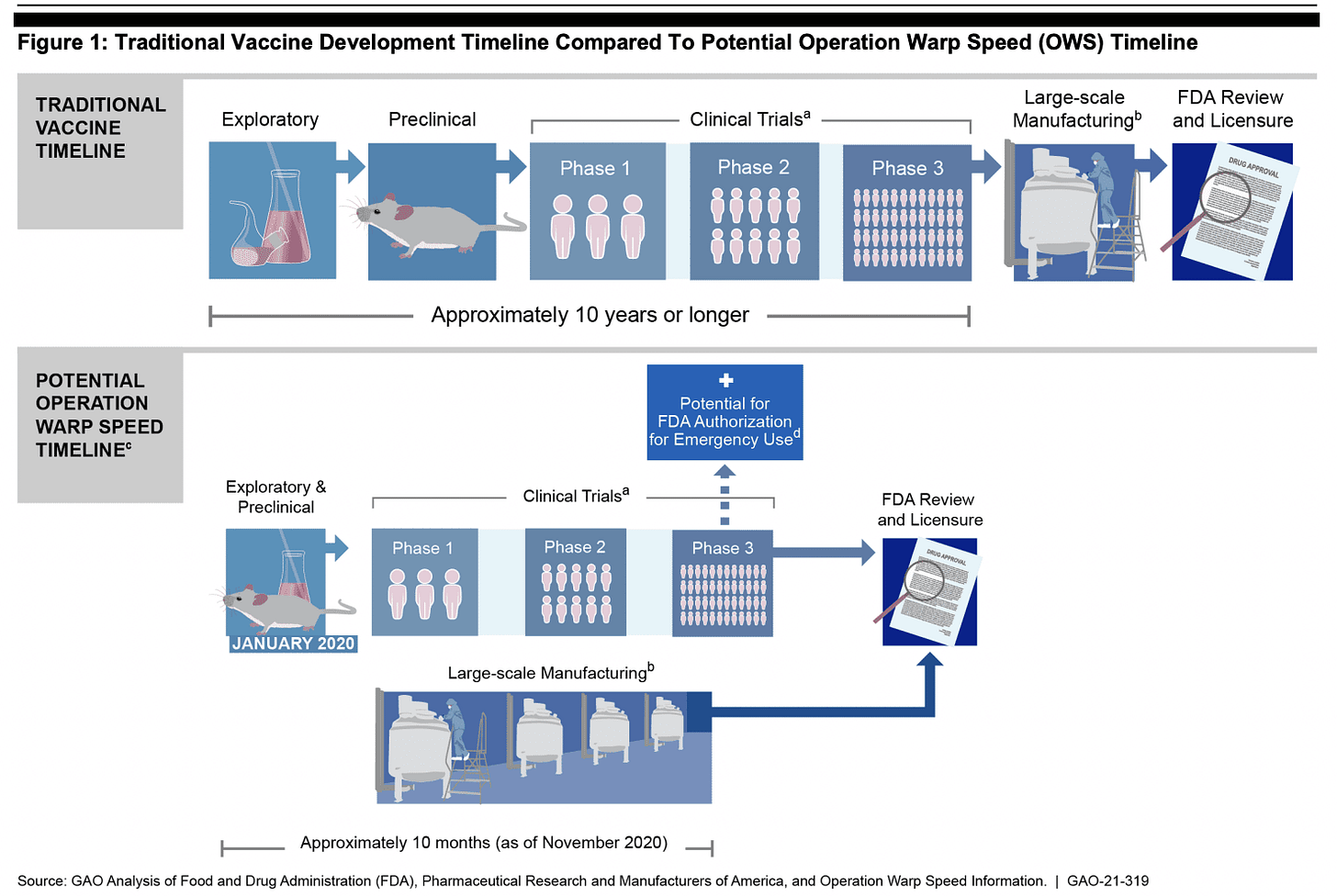
New pharmaceutical products usually undergo a 10 (or more) year development process. The covid-19 vaccines, which don’t meet the traditional definition of vaccines because they don't prevent infection or transmission, were brought to market in a mere 10 months, without animal studies or extended safety and efficacy studies. No independent (from manufacturer) studies are available. They are granted EUA use, and recipients are effectively part of the world’s largest clinical trial!
The World Council for Health have conducted an independent review of reported AEFI following covid-19 vaccination using VAERS (US), Vigiaccess (WHO), Yellowcard (UK) and Eudravigilance (European) systems. More than 40,000 deaths are linked as described in this press release, and as detailed in the main report. WCH concluded that there is sufficient evidence of severe harm and that a product recall should be made.
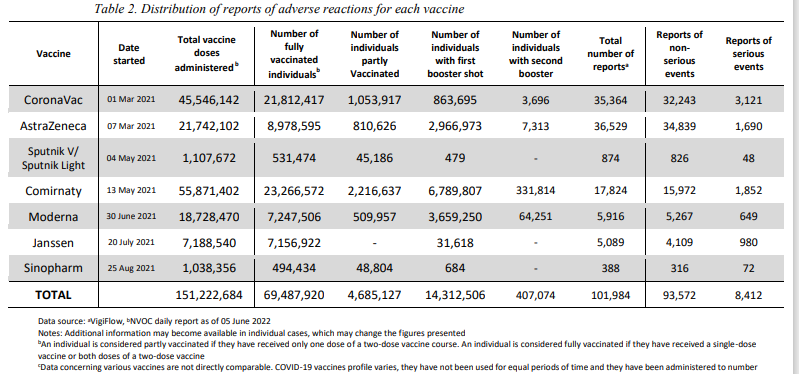
The latest Philippines FDA Pharmacovigilance report for the week ending 5th of June 2022 adds 828 new AEFI (new total of 101,984), including 169 serious reactions (new total serious to date is 8,412) of which 44 are deaths (new total 2,424 deaths to date). Serious reactions comprise 8% of reports; deaths comprise 29% of the serious reactions!
Pharmacovigilance systems are known to grossly underestimate population impacts, this is known as under reporting factor (URF). If my estimated minimal Philippines URF of 126 holds, these reported deaths could be just the tip of the iceberg with the 44 new deaths representing more than 5,500 possible new deaths, and the overall 2,424 deaths reflecting possibly well more than 300,000 AEFI deaths.
Philippines FDA still claim no signal for intervention or changing of the vaccination recommendations.
This is despite the FDA’s own guidelines for Drug recall which only require reasonable suspicion of harm or death! How many AEFI reports, how many deaths will raise that reasonable suspicion? How ironic, the FDA will recall cosmetic products and vitamin supplements, but won’t recall these EUA gene therapy drugs!
Link to the Philippines FDA Pharmacovigilance reporting system for Covid-19 Vaccination here. Anyone experiencing any suspected adverse reaction is encouraged to make a report. This is necessary for the gathering of population effects data.
Readers, please share this information, widely. Please discuss it with your families and friends, and submit it to your heath care professionals and government officials. Please use it for your own due diligence before you accept any (or any more) Covid-19 vaccination.





Hi Sally!
Sorry it's been a while since I last replied to one of your articles. After subscribing, google strangely needed a cellphone number to the account to sign in to your newsletter. I think I've skirted around this for now.
Anyway, I was perplexed looking at Table 2. Distribution of AE's from the Philippines FDA Pharmacovigilance report. I downloaded the report and indeed it states COMIRNATY!
I'm seriously troubled by this as there seems to be a deliberate attempt to bait with an FDA-approved shot but inject the Emergency Use Authorized product instead.
Source: https://pa.childrenshealthdefense.org/news/ghost-shot-pfizer-quietly-admits-it-will-never-manufacture-original-fda-approved-covid-vaccines/
-------
In May, Pfizer updated its statement to mention a December 2021 licensed Comirnaty product, which was granted a license four months after the highly-publicized August FDA press release.
And just last week, Pfizer finally acknowledged that its original licensed product will never be distributed. In an unreported update on the CDC website, Pfizer told the agency:
“Pfizer received initial FDA BLA license on 8/23/2021 for its COVID-19 vaccine for use in individuals 16 and older (COMIRNATY). At that time, the FDA published a BLA package insert that included the approved new COVID-19 vaccine tradename COMIRNATY and listed 2 new NDCs (0069-1000-03, 0069-1000-02) and images of labels with the new tradename. These NDCs will not be manufactured. Only NDCs for the subsequently BLA approved tris-sucrose formulation will be produced.”
-----------
I also observed that page 4 (Phil. FDA PharmaCoV report) lists the said shot as:
-----
At present, the FDA granted nine (9) COVID-19 vaccines with emergency use authorization:
● Pfizer-BioNTech COVID-19 mRNA Vaccine (nucleoside modified) [Comirnaty]
------
IT CANNOT BE BOTH THE EUA PFIZER SHOT AND FDA APPROVED COMIRNATY at the same time! This is clearly the Pfizer Shot under EUA.
What is this then? Dare I say that this report is fraudulent?