Philippines Pediatric AEFI. PH FDA Reports Augmented by VAERS Reports. PH-FDA deaths match VAERS deaths for 0-11 age group who only got Pfizer. VAERS deaths are lower for 12-17, who also got
Coronavac, not monitored by VAERS. Summary of the 0-11 deaths is provided. Were there Filipino pediatric hot lots?
The Philippines, in its final pharmacovigilance report shows 2,846 deaths. Of these, 55 are from children aged 12-17 years of age, and 17 are from children aged 5 - 11 years of age.
Covid-19 vaccination of children aged 12-17 years old began on 15 October 2021 for children with co-morbidities and on 2 November 2024 for all other children in this age group. The mostly commonly used vaccines was Pfizer, though some children took coronavac and Moderna. The FDA reports that there were 4,546 reports of AEFI including 473 serious reports.
Covid-19 vaccination of children aged 5-11 years old began on 7 February 2022 for children with co-morbidities and on 2 November 2024 for all other children in this age group. Comirnaty/Pfizer was the only approved product for this age group. The FDA reports that there were 2,230 reports of AEFI including 247 serious reports.
An assessment of the VAERS adverse reactions for children aged 5 - 11 from the Pfizer product should be revealing, as it shows how many reports were made by the FDA to Pfizer.
Using OpenVAERS, I searched for Covid Vaccine Only, Philippines, Age 0-11, Pfizer/Biontech. The search result showed 326 reports, 79 more than the FDA had listed as being serious. When searched by death there were 16 reports of deaths, one less than reported by the Philippines FDA. Only serious reports are required to be forwarded to the Manufacturers who then submit these to VAERS.
Time to death ranged from same day as the injection to 3-months post-injection.
Eleven of deaths were female and 5 were male.
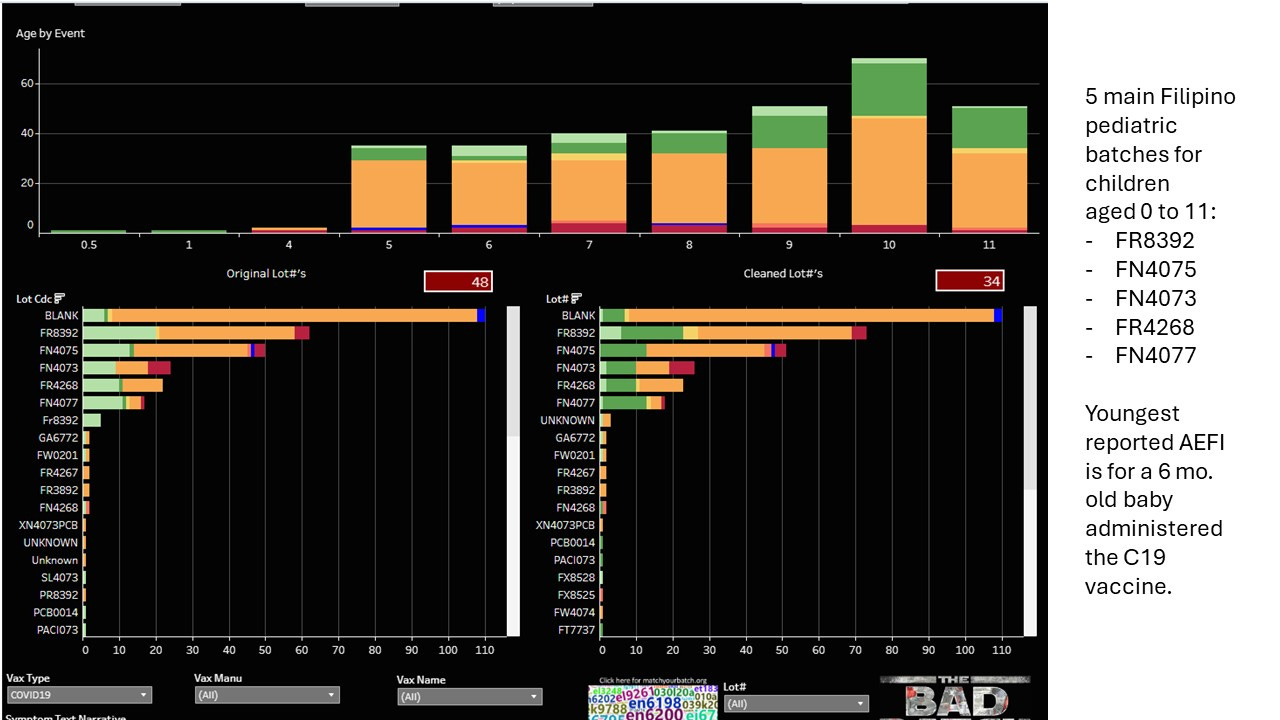
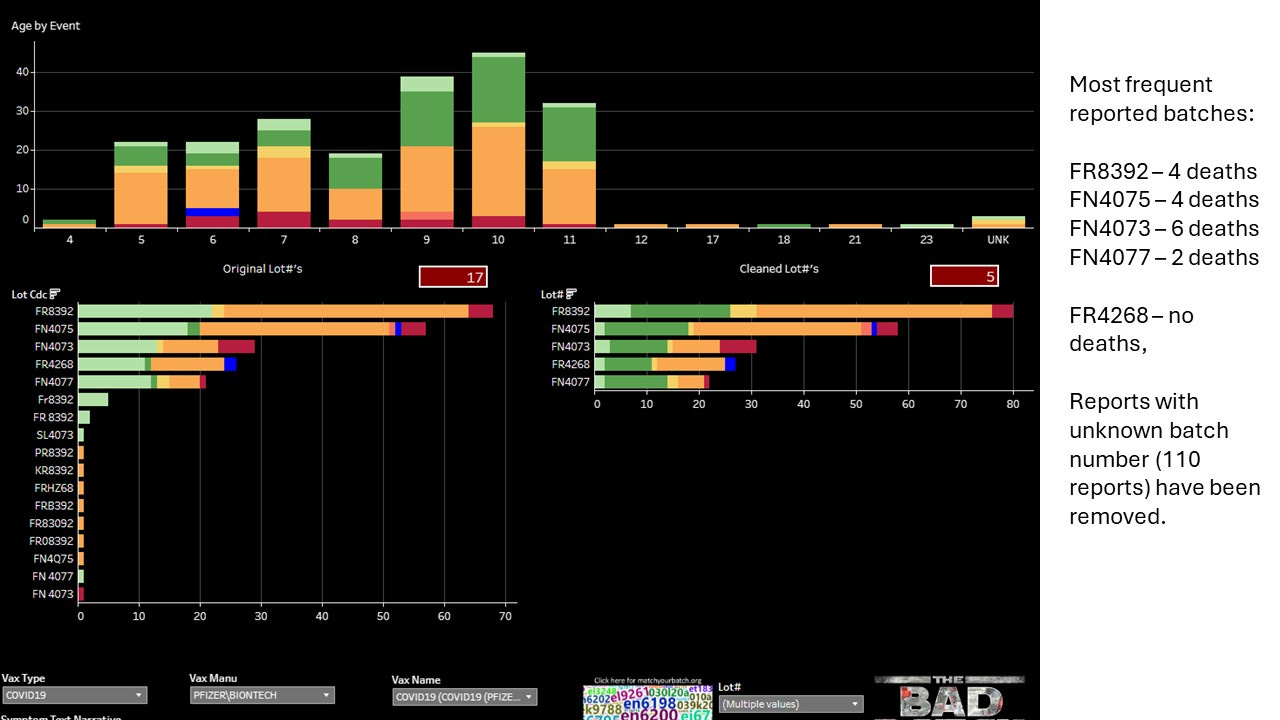
Batch No. FN4073 was associated with 6 of the deaths, FR8392 and FN4075 were each associated with 4 deaths, FN4077 was associated with 2 deaths. Do 6 deaths make a cluster? That will be a question for NAEFIC at the next hearing!
I have summarized these cases:
VAERS ID: 2183424. 7 yo female. Injected on 15 Feb 2022, lot no. FR8392. Outcome was reported as death, with no date or cause of death given. Ref. No.: PH-PHFDA-300137859. Report was received by VAERS on 17 March 2022.
VAERS ID: 2193609. 8 yo female. Injected on 18 Feb 2022, lot no. FR8392. Starting on 4th of March 2022, she experienced fever, headache, and abdominal pain. She experienced hematemesis (coughing blood), and cardiac arrest on 6th March, resulting in death. Ref. No.: PH-PHFDA-300138966. Death 16 days post injection. Report was received by VAERS on 23 March 2022.
VAERS ID: 2214565. 7 yo male. Injected on 23 March 2022, lot no. FN4075. Reported cough, and shortness of breath onset on 23 March, resulting in death. Ref. No.: PH-PHFDA-300141237. Same day death. Report was received by VAERS on 5 April 2022.
VAERS ID: 2249143. 11 yo male. Injected on 29 March 2022, lot no. FN4077. Reported cough on 30 March 22, colds and fever on 2nd April 2022, headache and shortness of breath onset on 4 April, resulting in death. Ref. No.: PH-PHFDA-300143230. Death 6 days after injection. Report was received by VAERS on 23 April 2022.
VAERS ID: 2268365. 7 yo female. Injected on 18 April 2022, lot no. FN4073. Diarrhea, fever, vomiting, seizure with onset on 18 April 2022. Fatal outcome. Ref. No.: PH-PHFDA-300145466. Same day death. Report was received by VAERS on 10 May 2022.
VAERS ID: 2316094. 6 yo female. Injected on 28 Feb 2022, lot no. FR8392. Upper abdominal pain, vomiting with onset on 28 May 2022. Fatal outcome. Ref. No.: PH-PHFDA-300148398. 3 months to death. Report was received by VAERS on 11 June 2022.
VAERS ID: 2334700. 10 yo female. Injected on 27 April 2022, lot no. FN4075. Malaise and fever with onset on 21 May 2022. Fatal outcome. Ref. No.: PH-PHFDA-300149589. 1 month to death. Report was received by VAERS on 25 June 2022.
VAERS ID: 2334704. 10 yo female. Injected on 27 April 2022, lot no. FN4073. Fever and headache with onset on 28 April 2022. Fatal outcome. Ref. No.: PH-PHFDA-300149743. 1 day to onset, resulting in death. Report was received by VAERS on 25 June 2022.
VAERS ID: 2373124. 5 yo female. Injected on 10 June 2022, lot no. FN4073. Nasopharyngitis with onset on 11 June 2022. Fatal outcome. Ref. No.: PH-PHFDA-300151017. 1 day to onset, resulting in death. Report was received by VAERS on 25 June 2022.
VAERS ID: 2395618. 10 yo female. Injected on 30 June 2022, lot no. FN4073. Diarrhea with onset on 8 July 2022. Fatal outcome. Ref. No.: PH-PHFDA-300152368. About 1 week to death. Report was received by VAERS on 1 July 2022.
VAERS ID: 2396993. 7 yo female. Injected on 19 April 2022 with dose no. 2, lot no. FN4073 (Dose No. 1 was FN4075 on 25 Mar 2022). Fever, abdominal pain with onset on 13 July 2022. Rash on 15 July. Seizure on 17 July with fatal outcome. Ref. No.: PH-PHFDA-300151887. About 1 month to death. Report was received by VAERS on 30 July 2022.
VAERS ID: 2415449. 8 yo female. Injected on 15 June 2022, lot no. FN4073. Septic shock, respiratory arrest with onset on 9th August 2022. Fatal outcome. Ref. No.: PH-PHFDA-300153937. About 2 months to death. Report was received by VAERS on 19 August 2022.
VAERS ID: 2423639. 9 yo male. Injected on 15 July 2022, lot no. FN4073. Depressed consciousness, headache, seizure with onset on 25th July 2022. Fatal outcome. Ref. No.: PH-PHFDA-300154077. About 10 days to death. Report was received by VAERS on 30 August 2022.
VAERS ID: 2428296. 6 yo male. Injected on 22 July 2022, lot no. FN4075. Fever, diarrhea, fever, headache, seizure with onset on 22th July 2022. Difficulty breathing on 27 July 2022. Fatal outcome. Ref. No.: PH-PHFDA-300154541. Same day onset. Report was received by VAERS on 3 Sept 2022.
VAERS ID: 2448931. 8 yo male. 12 Mar 2022 Dose 1 FN4077. 4 April 2022, lot no. FN407? for dose 2. Fever with onset on 1st May 2022. Fatal outcome. Ref. No.: PH-PHFDA-300155315. About 4 weeks to onset. Report was received by VAERS on 17 Sept 2022.
VAERS ID: 2591250. 9 yo female. 29 Mar 2022 Dose 1 FR8392. Upper abdominal pain, fever with onset on 12 April 2022. Fatal outcome. Ref. No.: PH-PHFDA-300162428. About 2 weeks to onset. Report was received by VAERS on 3 March 2023.
When I cross reference using the VAERSAWARE platform, there are 327 reported reactions and 17 deaths [I haven’t been able to find the VAERS number/report for this death], this is 100 fewer than the number of AEFI reported by the Philippines FDA for this age-group but matches the number of reported deaths.
There are 5 main batches given to Filipino children aged 5 to 11; 4 of these have reports of deaths.
This shows key common symptoms reported as experienced by the children.
Relative toxicity is shown.
I wanted to see if these batches were exclusive to the Philippines. To do this I removed the reports with unknown lot numbers (110 reports). This left 217 reports. The majority of reports did come from Philippines, with just a very small handful (15) coming from other countries.
Batch FR4268 did not have reports for any country other than the Philippines, though it had one unknown foreign report.
CHILDREN AGED 12 - 17 PHFDA VS. VAERS
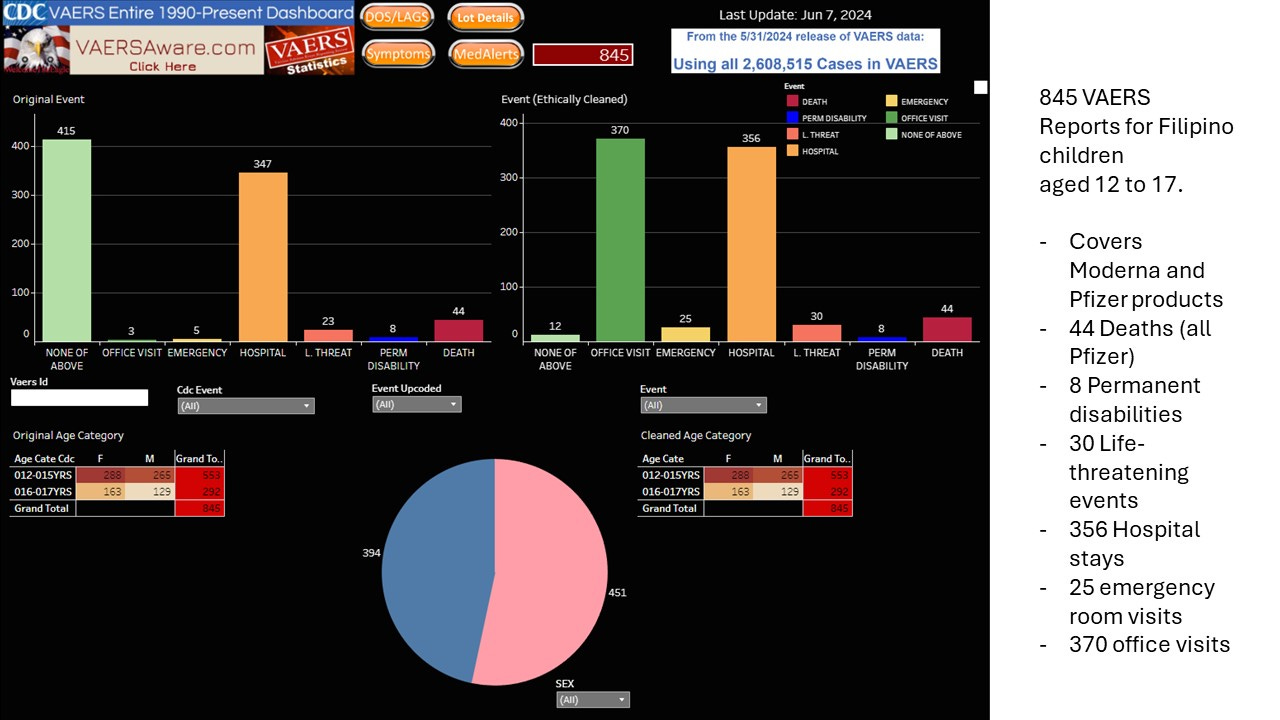
For older children, aged 12 to 17, the Philippines FDA reported 473 serious outcomes.
From VAERSAWARE, there are 845 reports of serious outcomes (372 more than reported by PHFDA), including 44 deaths and 356 hospitalizations. This contrasts with PHFDA which reports 55 deaths in this age-group. However, the 11 additional deaths could be due to another product such as the Chinese Coronavac, which the VAERS system does not capture:;as it only reports US Manufacturer data. All reports for children aged 12 - 17 are related to Pfizer and Moderna. All reported deaths were due to Pfizer.
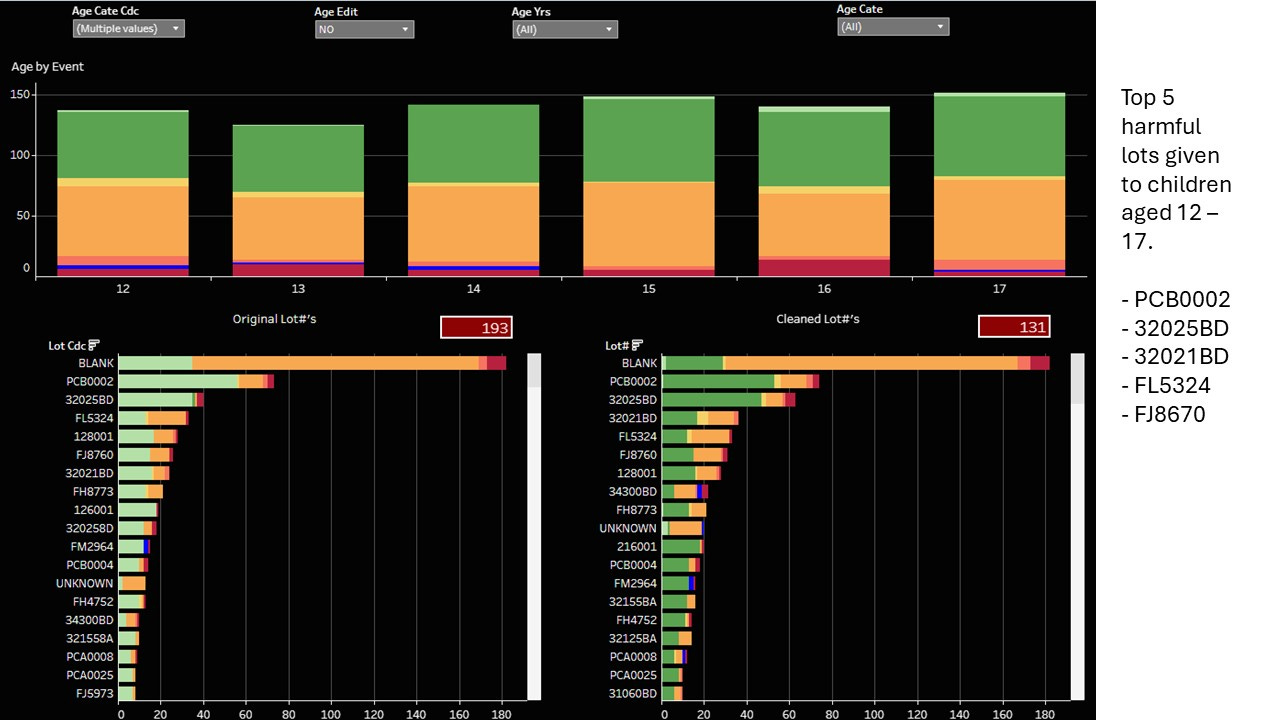
The top 5 harmful lots are listed.
Key symptoms are different from those in the younger children.
CONCLUSION
An evaluation of pediatric adverse reactions in the Philippines using the Philippines FDA, OPENVAERS, and VAERSAWARE gives a picture of vaccine outcomes.
The deaths reported for young children 0-11 in the PHFDA reports matches the VAERS reports of deaths (17).
The deaths reported for children aged 12-17 by the PHFDA is higher than that reported by VAERS (44), probably due to non-US product deaths.
There are frequent, but different reported symptoms, for the different age-groups.
There were 5 lots given to Filipino children aged 5-11, which seem to be nearly exclusive to the Philippines; only 2 deaths and 15 total reports outside of the Philippines. Were Filipino children targeted as a specific experimental group?
Given the very low risk to children from Covid-19 infections, the reported adverse outcomes do not support benefit of these injections outweighing risks. These reports may well be only the tip of the iceberg of harm to children given the known massive underreporting in the Philippines which is surely far greater than the international URF of 40 - 100.
Further, my local news feeds are full of sudden deaths of young and working age people, including children. This is not over yet.













Thank you for the thorough analyses with even limited data.
This is what I don't understand- very early on, it was already clearly stated even by the US CDC that children and youngsters were not at risk at all for the so-called "COVID" whatever-it-was (a bioweapon, in my view). So why were they pushing these toxic shots on young people? Doctors, nurses, health center people who gave all these were all so ignorant, or blind, or brainwashed? Contrary to all the official pronouncements even?
What a giant evil program that was deployed upon the ignorant, unthinking, and brainwashed.
Excellent use of vaesaware.com
God Bless Ms. Sally!